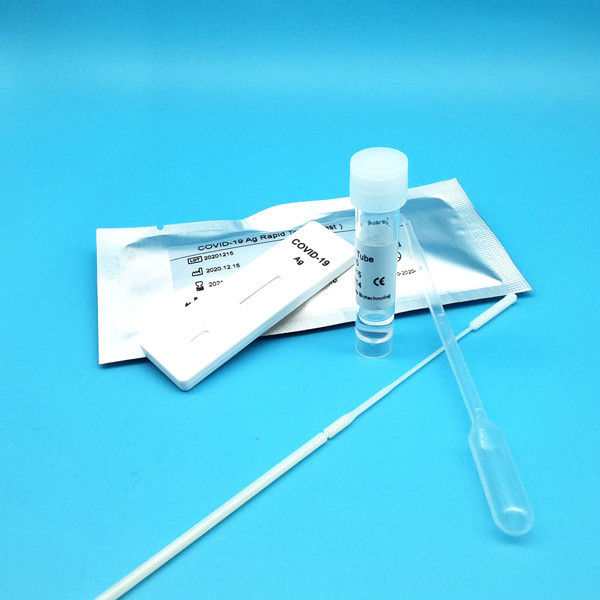
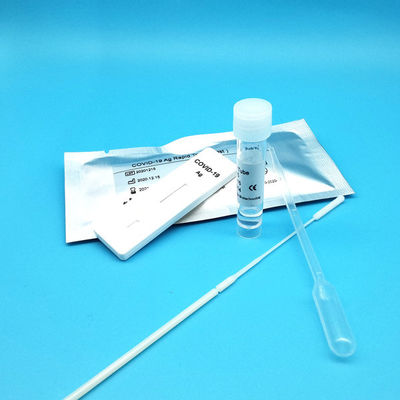
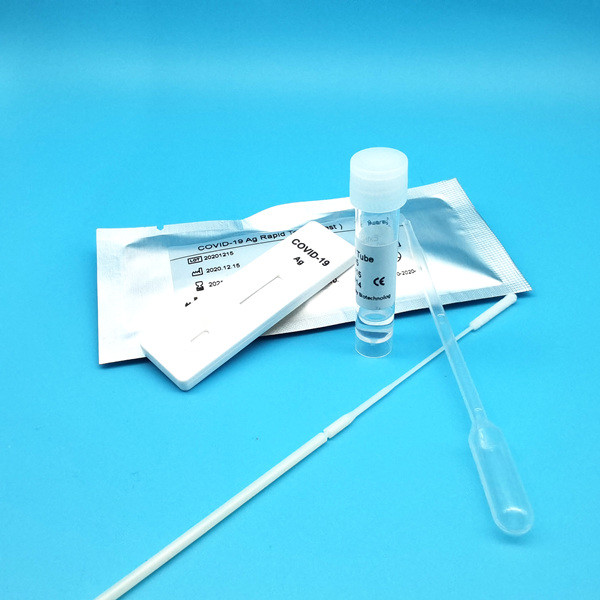
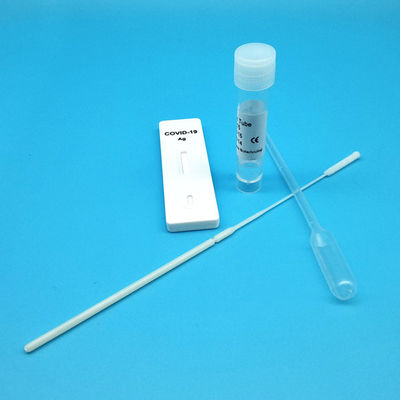
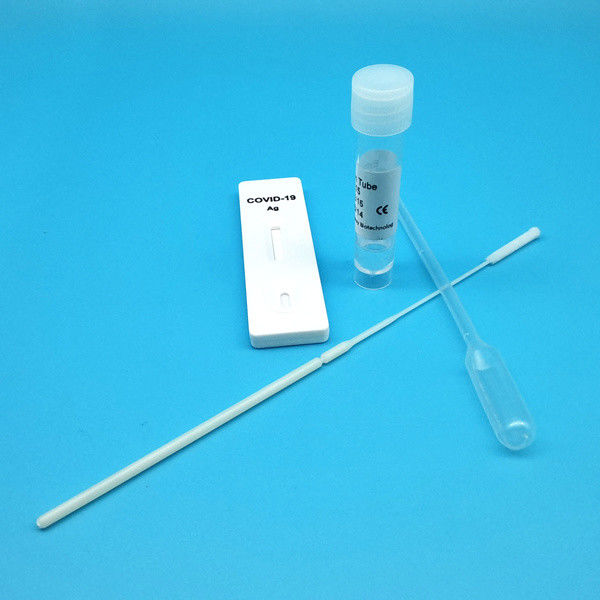
Product Description
One-Step Home Use Rapid Test Kit With Buffer For Testing
【INTENDED USE】
The Ag Rapid Test is an in vitro immunochromatographic assay for the qualitativ detection of Influenza antigen in a test device from nasal swab, throat swab or nasal wash/aspirate specimens. It is intended to aid in the rapid differential diagnosis of influenza SARS-CoV-2 viral infections. This test provides only a preliminary test result. Therefore, any reactive specimen with the Ag Rapid Test must be confirmed with alternative testing method(s) and clinical findings. The test is intended for professional and laboratory use.
【INTRODUCTION】
Corona virus belongs to coronaviridae, nidovirales and is divided into α, β and γ genuses. α and β genuses can only make mammal sick, and γ genus mainly causes birds to be infected. is mainly transmitted through directly contacting secreta or transmitted by aerosol or spray. But there is also evidence showing it can be transmitted by fecal-oral route. By now, there has been already up to 7 types of HCoVs that can cause human respiratory disease: HCoV-229E, HCoV-OC43, SARS-CoV, HCoV-NL63, HCoV-HKU1, MERS-CoV and . So HCoV is a major causative agent to cause human respiratory tract infection. It can evolve into severe pneumonia, respiratory failure, acute respiratory distress syndrome, septic shock, multiple organ failure and severe acid-base unbalance, etc. and even threaten life. Rapid diagnosis of SARS-CoV-2 infection will help healthcare professionals to treat patients and control the disease more efficiently and effectively.

【MAINCOMPONENTS】
Test Device (25): Each foil pouch contains one single-use test device with one membrane strips. The strip contains a test line of monoclonal
Extraction Tube(25): One tube containing 0.5ml extraction solution.
Dropper (25)
Sterile Nasal Swab(25)
【STORAGE INSTRUCTIONS】
1. Store at room temperature (2-30℃ or 35.6-86°F) in a dry place. Avoid direct sunlight;
2. 12 months of shelf life (date of manufacture to expiration date).
【TESTPROCEDURE】
Step 1 Bring the test to room temperature (18-26℃) before use.
Step 2 Open the foil pouch, take out the test and lay it on an even surface.
Step 3 Add 5 drops(about 80 µL )of extraction mixture into the each sample well (S).
Step 4 Read and record the results at 15 minutes after addition of samples.
NOTE: Result will be invalid after 20 minutes. After observing and recording the result, dispose of bio-hazardous materials by following the practices of your institution. Discard all the materials in a safe and acceptable manner in compliance with all the federal, state, and local requirements.

【EPLANATION OF TEST RESULTS】
1. Negative result: if only quality control line C appears and test line T show no color, it means no Influenza antigen is detected and the test result is negative (as shown in the following figure).

2.Positiveresult:If the third strip both quality control line C and T appear, it means the antigen is detected and the test result is the antigen positive (as shown in the following figure)

3. Invalid result: if quality control line C can not be observed, regardless of test line, the result would be invalid.(as shown in the following figure) The sample should be tested again.

NOTE
1.The intensity of color in the test line (T) may vary depending on the concentration of analyses present in the specimen. Therefore, any shade of color in the test line (T) should be considered positive. Please note that this is a qualitative test only, and cannot determine the concentration of analyses in the specimen.
2.Insufficient specimen volume, incorrect operating procedure or expired tests are the most likely reasons for control band failure.
【PERFORMANCE CHARACTERISTICS】
1. Negative reference coincidence rate
Use 10 samples of Ag negative reference to test, the test results should not be positive, the negative coincidence rate should be 10/10;
2. Positive reference coincidence rate
Use 10 samples of Ag positive reference to test, the test result should all be positive, the positive coincidence rate should be 8/10;
3. Sensitivity
Use 3 samples of Ag sensitivity reference to test, L1 should be detected, L2 should be detected or not,L3 should not be detected.
4. Repeatability
Use 1 sample of internal control repeatability reference( J1) to complete the parallel test for 10 tests, the results should be coincident.
5. Cross-reaction and interference reaction
Use positive samples of Rhinovirus, RS virus, Influenza A virus, Influenza B virus, Chlamydia, Mycoplasma and Bacterial Infection to do the tests, the test results should be negative.
6. The Clinical study
The Ag Rapid Test has been evaluated with 155 clinical samples. Both 91 negatives and 64 positives were confirmed by PCR
The Ag Test v.s. PCR
|
Test Method
|
Viral culture results |
Total
|
| Positive |
Negative |
| The Ag Test |
Positive |
52 |
7 |
59 |
| Negative |
12 |
84 |
96 |
| Total |
64 |
91 |
155 |
[1] Sensitivity = A/(A+ C) ×100%= 81.3%
[2] Specificity = D/(B+ D) ×100%= 92.3%
[3] Crude coincidence rate= (A+D)/(A+B+C+D)×100%= 87.7%
[4] Consistency factor kappa = 0.744
【EXPLANATION OF SYMBOLS ON LABEL AND PACKAGE】


 Your message must be between 20-3,000 characters!
Your message must be between 20-3,000 characters! Please check your E-mail!
Please check your E-mail!  Your message must be between 20-3,000 characters!
Your message must be between 20-3,000 characters! Please check your E-mail!
Please check your E-mail!